MDCG 2024-12 Rev.0
Corrective and preventive action (CAPA) plan assessment: guidance and templates for conformity assessment bodies, notified bodies, designating authorities and joint assessment teams
Disclaimer: This document is an interactive version of the original MDCG document. We will keep it up-to-date.
This document has been endorsed by the Medical Device Coordination Group (MDCG) established by Article 103 of Regulation (EU) 2017/745. The MDCG is composed of representatives of all Member States and it is chaired by a representative of the European Commission.
Table of Contents
1 Introduction
This guidance document is intended for conformity assessment bodies (CABs), notified bodies (NBs), designating authorities (DAs), and Joint Assessment Teams (JATs) involved in Regulation (EU) 2017/745 on medical devices (hereafter MDR) and Regulation (EU) 2017/746 on in vitro diagnostic medical devices (hereafter IVDR). It should be read in conjunction with the guidance document MDCG 2022-13 “Designation, re-assessment and notification of conformity assessment bodies and notified bodies” (1).
This document aims to provide guidance for:
− NBs (2) when establishing the corrective and preventive action (CAPA) plan to address the non-compliances (NCs) resulting from joint assessments according to Article 39(5) of the MDR or Article 35(5) of the IVDR,
− authorities responsible for notified bodies (hereafter, the DAs) when conducting reviews of and providing opinions on CAPA plans of notified bodies according to Article 39(7) of the MDR or Article 35(7) of the IVDR and
− JATs when considering the CAPA plan and the DA’s opinion thereon according to Article 39(7) of the MDR or Article 35(7) of the IVDR.
The use of the templates in Annex I (hereafter CAPA template) and Annex II (hereafter JAT review template) to this guidance is not mandatory. However, using them according to this guidance to structure CAPA plans and conduct their reviews will facilitate an efficient, consistent and timely CAPA review process for NBs, DAs and JATs.
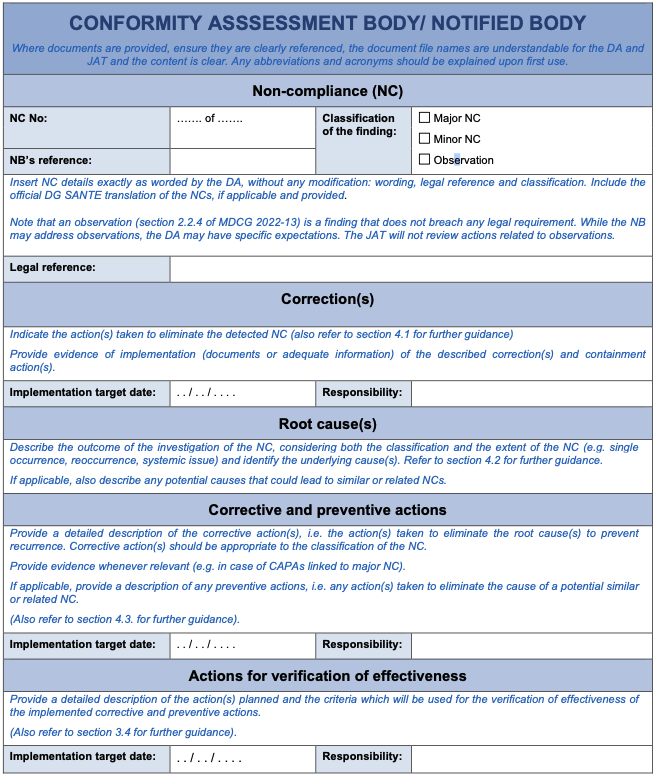
The formal list of non-compliances from the on-site assessment provided by the DA serves as the input to the CAPA process. When completing the CAPA template, the wording, legal references, and classification of the NC(s) should be restated without modification. This includes the official DG SANTE translation of the NC, if applicable and provided (3).
Clear and traceable communication throughout the CAPA process is crucial to ensure an efficient review by the JAT of CAPA plans confirmed by the DA, as well as the DA’s opinion regarding those CAPA plans, ultimately facilitating the JAT’s final opinion.
2 Scope
This document provides guidance for CABs, NBs, DAs and JATs on using the templates in Annex I and Annex II during assessments of NBs and CABs under the MDR and the IVDR.
These templates are primarily designed for re-assessments of NBs. However, they can also be applied during assessments of CABs applying for designation as an NB, assessments relating to extensions of an NB’s scope of designation, and assessments relating to challenges to an NB’s competence under Article 47 MDR or Article 43 IVDR. While this guidance document provides comprehensive information, it’s important to note that specific guidance may not be applicable in every context. For example, the requirement for containment actions may not be relevant during designation assessments (see Section 4.1).
The CAPA template is not designed to be used by DAs to document the NCs raised during assessments as DAs may have their own template for this purpose. However, if it suits their needs, DAs may use it for this purpose too.
3 Timelines of the process
The timelines for the process are described in MDCG 2022-13. Stakeholders should familiarise themselves with that guidance and aim to implement the timelines described.
4 Considerations for the NB
If the DA has not already done so, the NB should transfer all NCs from the DA’s assessment report/list into the CAPA template. This should be done exactly as stated and including legal references, classification and the official DG SANTE translations of the NCs, if applicable and provided.
It is recommended that the NB completes the relevant sections of the CAPA template in as much detail as possible, focusing on providing clear, comprehensive and appropriate information to enable the DA and JAT to effectively review and make an informed assessment of the information provided. The NB may also consider providing supporting evidence (e.g. updated procedures, new templates, …) together with the completed CAPA template, if deemed helpful for the understanding and assessment of the information provided by the NB in the CAPA template.
The NB should assign a person responsible for implementing corrections, corrective and preventive actions and actions to verify their effectiveness. This should be documented in the template along with the target date(s) for the implementation.
The completed CAPA plan and, where appropriate, supporting evidence should be sent to the DA by the timeframe communicated by the DA (4).
If the DA has classified a finding as an observation (5) and communicated specific expectations, the NB is encouraged to meet these expectations as part of ensuring effective CAPA management. Additionally, the NB may consider taking steps to address any observation which could involve improving the current situation or implementing preventive measures to avoid similar issues in the future.
4.1 Corrections
In this section of the CAPA template, the NB should provide a detailed description of all corrections, whether they are containment actions or not.
A ‘correction’ is an action to correct or eliminate a detected NC in whole or in part. (6)
An ‘containment action’ (also called a ‘’immediate correction’) is a correction that the NB should take without any unjustified delay to address an identified risk or safety issue, aiming to control the situation and prevent further (potential) harm (7). Examples which may require a containment action include cases when the DA or JAT detected failure of the NB to adequately assess the validation of the sterilisation process, a certificate which the NB issued having overlooked an open major non-conformity impacting the device safety or performance, or a certificate issued out of the scope of designation (and competence). Containment actions may include for example immediate restriction of the scope of a certificate or suspension of a certificate.
Upon identification of an NC, the NB’s first step is to consider and, where appropriate, implement one or more containment actions. Additional corrections may be deemed necessary following the full investigation of the NC.
The NB should also provide evidence (documents or adequate information) of the implementation of these corrections, where appropriate: particularly for containment actions and corrections deemed necessary by the DA.
The NB should consider the potential impact of each correction on its quality management system (QMS) as a whole. This includes:
- The impact on other documents, processes or procedures. For example, if the revision of a deficient procedure has an impact on other procedures/processes, all these procedures/processes should be reviewed, assessed and revised as appropriate.
- The impact on other conformity assessment projects and existing certificates. For example, if a deficient sterilisation checklist has had a critical impact on how the NB has assessed ‘sterilisation’ in its conformity assessment projects, not only the project in which the finding was raised as an NC, but all those projects affected by the same deficient checklist should be reviewed, assessed and corrected as appropriate.
- The identification of similar shortcomings. The NB should identify issues in other parts of the QMS, even before the root cause is determined. For example, if a deficient checklist for assessing the validation of the sterilisation process is identified, the NB should also consider whether checklists used for the assessment of other sterilisation methods have the same or a similar weakness. If so, potentially affected projects using those checklists might also require review and correction.
4.2 Root cause(s)
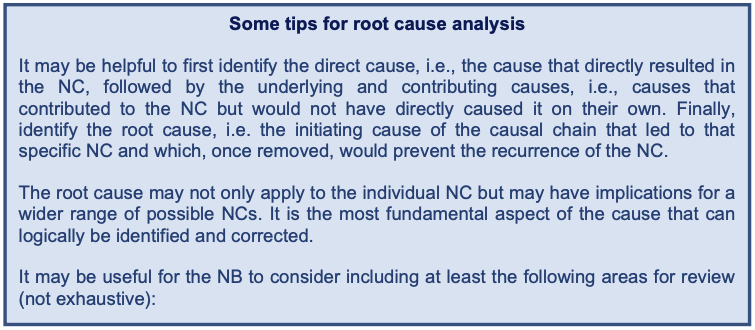
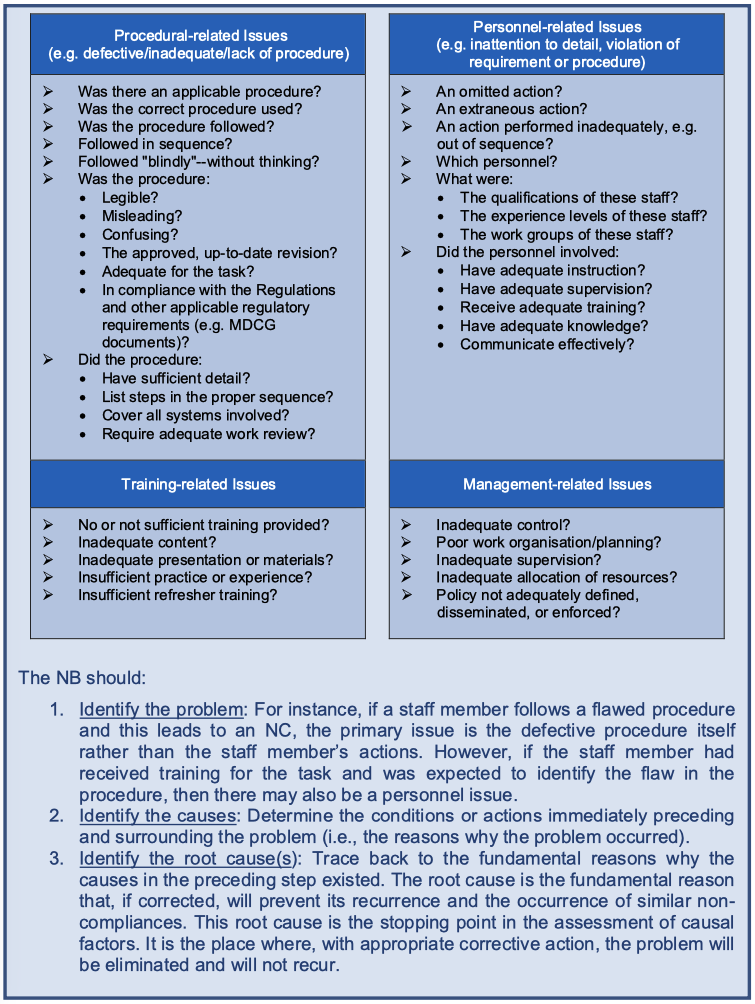
The core issues that caused the NC should be identified through a comprehensive root cause analysis. The NB should use a set of analysis and problem-solving techniques aimed at investigating in detail the NC, considering both the severity (e.g. major or minor) and the extent of the NC (e.g. single occurrence, reoccurrence, systemic issue). The root cause analysis should identify the actual root causes or the reasons that caused the NC and not just how to eliminate the symptoms of the issue (8). It should be noted that there may be more than one (root) cause for an NC.

4.3 Corrective and preventive actions
This section in the CAPA template focuses on corrective and preventive actions. As defined in Article 2 (67) MDR and Article 2 (70) IVDR, a corrective action is ‘any action taken to eliminate the cause of a potential or actual non-conformity or other undesirable situation’. In case of an actual NC, CAPAs aim to prevent recurrence. The NB should select CAPAs that are appropriate to the classification and severity of the NC. These CAPAs should be comprehensive, outlining the actions identified to address the root causes and should describe any related preventive actions. For major NCs, the NB should also demonstrate evidence of implementation for both corrective and preventive actions.
CAPAs consist of improvements to the NB’s processes to eliminate the causes of NCs, to correct and eliminate recurring NCs and to prevent a potential NC from occurring. The corrective actions identified to eliminate the causes of the NC should be clearly linked and consistent with the causes identified in the root cause analysis section. Corrective actions should address systemic issues. For example, simply changing a procedure and providing training to personnel on the revised procedure may not be appropriate or sufficient to address systemic issues that may have contributed to the NC.
When identifying root causes and corrective actions, the NB should also consider whether potential NCs have not (yet) been identified and take preventive actions to avoid them. For example, if one of the causes was a lack of in-depth knowledge of a sterilisation method by the author of the sterilisation procedure, the NB should review other sterilisation procedures written by the same author and act (if appropriate), even if no NC was raised regarding these other procedures during the joint assessment. This approach ensures a comprehensive review of potential issues.
In general, root cause analysis, corrections, corrective and preventive actions should address the actual NC and associated potential NCs throughout the NB’s QMS (e.g. site-specific procedures; amended SOP in one language which was not translated, leading to discrepancy between the same SOP in different languages).

Distinguishing between correction, corrective action, and preventive action can be challenging, depending on the root cause. This document serves as a guide, not a rigid set of rules. When unsure about categorising an action when completing the CAPA template, it is suggested to primarily focus on its effectiveness in enhancing the system and include the action only once in the section in the CAPA template where the NB thinks it fits the best. In the above-mentioned example of a deficient checklist for assessing the validation of the sterilisation process: some of the actions can be considered a corrective action and/or a preventive action instead of a correction, depending on the root cause.
4.4 Actions for verification of effectiveness
In this section of the CAPA template, the NB should detail the planned actions, including responsibilities and timelines, to verify the effectiveness of the implemented corrective actions and, where appropriate, preventive actions. The NB should define SMART criteria (Specific – targeting the identified root cause(s), Measurable, Achievable, Realistic and Time-bound) to determine if the NC has been effectively addressed. The NB should establish appropriate timeframes for scheduling effectiveness checks, considering the classification of the NC, the complexity of the implemented corrective actions and, where appropriate, the complexity of the implemented preventive actions. The timeframe should allow sufficient time for the implemented actions to take effect, typically ranging from several months to a year. Progress may be assessed during scheduled internal audits.
The NB should verify and demonstrate that:
− the cause has not recurred,
− the NC has not recurred,
− a similar NC has not occurred and
− the corrective action remains effective and continues to be implemented.
The NB should document the results of its verification of effectiveness in a clear and accessible manner for the DA.
While the actions planned and the criteria which will be used for the verification of effectiveness are part of and should be documented in the CAPA plan, the verification of effectiveness itself falls outside the scope of the joint assessment and will be followed-up under the DA’s monitoring activities.
5 Considerations for the DA
It is important that the DA completes the relevant assessment section of the CAPA template in sufficient detail to ensure their assessment and opinion on the CAPA plan are clear and can be fully understood by the JAT, minimising the need for further requests for clarification.
The process outlined in Section 2.3.2 of MDCG 2022-13 should be followed for the DA’s assessment of the CAPA plan.
If a finding was classified by the DA as an observation, the DA should clearly communicate their expectations on how the NB should address the observation.
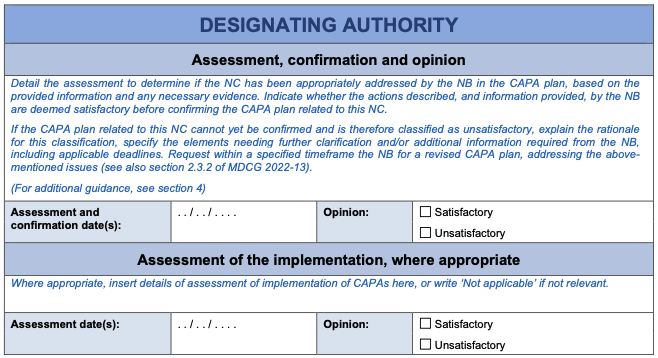
After confirming the corrective and preventive action plan, if necessary following further successive requests for clarification or modifications from the NB, the DA should forward it to the JAT, together with its opinion thereon (9).
After receiving the JAT’s subsequent review, documented in the appropriate template (Annex II), the DA should carefully consider the JAT’s input, finalise their overall assessment of the CAPA plan, and provide feedback to the NB on JAT’s review and any follow-up steps.
If the JAT has requested further modifications, the DA may request a further update of the CAPA plan from the NB, as appropriate, and update its assessment in the CAPA template.
An iterative process may result from this. The NB may update the CAPA plan (if applicable) and the DA may revise its assessment of this CAPA plan. This may result in a further request for clarification and/or modifications from the JAT. This process continues until both parties, DA and JAT, reach agreement on the CAPA plan which should ideally be reached early in the process. The assessment of its implementation can subsequently follow, possibly involving further iteration, before reaching agreement on the consideration of the NCs being satisfactorily addressed.
For actions in the CAPA plan requiring implementation prior to the DA’s final report, the DA should document the verification (including identification of evidence, review and conclusions) in the CAPA template. This includes identifying the evidence reviewed by the DA and confirming that the actions were successfully implemented by the NB. Based on experience, providing relevant documentation from the NB demonstrating the implemented actions, along with DA’s assessment, to the JAT during the CAPA assessment phase can help avoid a negative JAT final opinion to the MDCG. Alternatively, if providing such documentation is impractical or not considered necessary, a more detailed description of the DA’s assessment of the implementation should be included in the template. This should include an accurate description of the actions, sufficient for the JAT to consider if it concludes with the DA’s assessment, e.g., that all the major NCs are resolved.
6 Considerations for the JAT
The JAT completes the JAT review template after receipt of each update of the CAPA plan, so that the JAT’s appraisal of the CAPA plan and of the DA’s opinion thereon is clear and can be fully understood by the DA. This includes any explicit request for further clarifications and modifications of the CAPA plan.
For each NC, the JAT should indicate whether it:
− accepts the CAPA plan, including the root cause(s), the correction(s) and corrective action(s), in line with the requirements of the Regulation and agrees with the DA’s assessment of it, or
− requests further clarification on the CAPA plan (e.g. the CAPA plan describes that a specific procedure will be updated, however detailed information on the planned changes in this procedure is missing), and/or
− requests for modifications of the CAPA plan when it considers any corrections, root causes, corrective actions or other aspects to be unacceptable, insufficient or inadequate. These requests will be clearly documented and motivated in the JAT review template.
The JAT will not review any of the NB’s actions related to observations, nor any of the DA’s opinions on them, as observations themselves do not breach a legal requirement.
Annex I: Template CAPA plan and assessment thereon
PART I: Basic information

PART II: CAPA plan and assessment thereon (to be copied and completed for each NC)
Annex II: Template JAT review of the CAPA and the DA’s opinion
The JAT will indicate whether it agrees with the DA’s opinion, if clarifications are needed, or if any of the actions are not deemed as acceptable and therefore a modified CAPA plan should be submitted. An explanation should be provided in the latter cases.
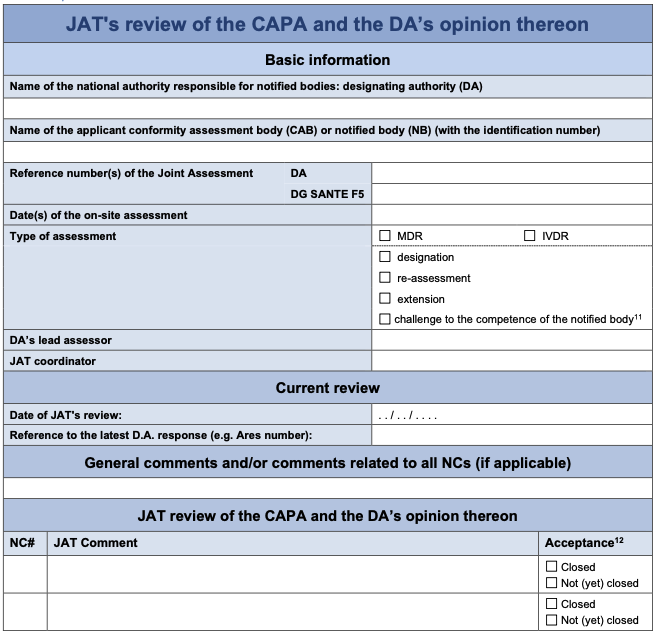
This is the original MDCG Template that is part of the MDCG 2024-12 document Rev. 0.
Footnotes
(2): Reference to NBs throughout this document may also be considered relevant to CABs.
(3): MDCG 2022-13 describes the official DG SANTE translations of the NCs in the last paragraph of section 2.2.5.
(4): Language considerations for CAPA plans are addressed in sections 2.3.2 and 2.3.3 of MDCG 2022-13.
(5): An observation (MDCG 2022-13, section 2.2.4) is a finding requiring attention from the NB but does not breach a legal requirement. MDR/IVDR and MDCG 2022-13 are silent on specific actions for observations.
(6): ISO 9000:2015 Clause 3.12.3, modified.
(7): While ‘containment action’ and ‘immediate correction’ are common terms in quality engineering, they are not explicitly defined in the MDR, regulatory guidance or relevant ISO standards. For example, the 8D method, a well-known quality problem-solving approach, describes these concepts.
(8): The European standard EN 62740:2015 describes a structured approach for root cause analysis (RCA), selecting appropriate techniques, and understanding their strengths and weaknesses. While not specific to medical devices nor notified bodies, it offers valuable principles for RCA within quality management systems.
(9): MDR Article 39 (7) / IVDR Article 35 (7)
(10): Article 47(3) MDR or 43(3) IVDR assessment
(11): Article 47(3) MDR or 43(3) IVDR assessment
(12): See section 6 in the guidance: if ‘Closed’, the JAT accepts the CAPA plan and the DA’s opinion thereon for this NC. In the other case, the JAT motivates a request for further clarification or modification of this CAPA plan.
