MDCG 2023-3 Rev.2
Questions and Answers on vigilance terms and concepts as outlined in the Regulation (EU) 2017/745 on medical devices
Disclaimer: This document is an interactive version of the original MDCG document. We will keep it up-to-date.
This document has been endorsed by the Medical Device Coordination Group (MDCG) established by Article 103 of Regulation (EU) 2017/745. The MDCG is composed of representatives of all Member States and it is chaired by a representative of the European Commission.
MDCG 2023-3 Rev.2 changes
MDCG 2023-3 (February 2023) MDCG 2023-3 Rev 1 changes (October 2024) | ||
|---|---|---|
| General | Questions are renumbered from Q.3 onwards due to the addition of new questions. The term ‘Regulations’ and references to IVDR articles are inserted, where relevant, throughout the document. Adjustments all over the document to align it to Regulation (EU) 2017/746 on in vitro diagnostic medical devices (IVDR). Inclusion of a table of contents to provide readers with a general overview of the document. | |
| Introduction | Newly added sentences in the third paragraph starting with: Unless quoted directly from the legal text or otherwise specified…’ and ‘Furthermore, references to ‘the Regulations’ should be understood to cover both the MDR and the IVDR.’Footnote 1 amended to include a reference to Directive 98/79/EC and the newly added text: ‘is obsolete under the Regulations.’ | |
| Question 1 | Newly added sentence in the first paragraph: ‘The definitions of an incident and a serious incident are outlined in the table below.’ Newly added table outlining the definitions of an incident and a serious incident. Paragraphs three and four amended accordingly to avoid repetition of the definitions provided in this table. Footnote 4 amended to include following new sentence: Flowchart amended to include relevant terms and definitions mentioned in the IVDR. Reference to ‘flowchart 1’ removed. First paragraph under ‘Reportability under the MDR’, first sentence rephrased: | |
| Question 2 | Criterion A: Two newly added examples specific to IVDs. Removal of the following sentence: ‘including impairments leading to diagnosed psychological trauma’. The order of ‚permanent or temporary‘ in the sub-bullet point has been reversed. Three newly added examples of a serious public health threat have replaced previous examples. Last paragraph under criterion B, newly added sentence starting with: | |
| Question 3 | First paragraph, newly added sentence starting with: ‘For IVDs, the performance of a device (Article 2(39) IVDR) consists of the analytical and,…’ Newly added IVD- specific examples. | |
| Question 4 | Following previous sentence starting with ‘or persons installing or maintaining the device’ is amended as follows: ‘or persons responsible for installing or maintaining…’ Following sentence has been deleted: | |
| Question 5 | Last paragraph, sentence amended as follows: ‘Any information related to abnormal use of a device that reaches the manufacturer, including from healthcare professionals or through other post-market surveillance activities, must be documented and managed within its quality management system.’Footnotes 16 and 17 amended to complement existing text. | |
| Question 6 | First paragraph, newly added text, starting with: ‘‘Use-error due to ergonomic features’ can be described as...’Third paragraph, newly added text, starting with: ‘are not caused by factors such as user negligence…’Fifth paragraph, newly added text, starting with: ‘or devices used in therapeutic applications,…’ | |
| Question 7 | The question has been renumbered (previously question 3). The text has been slightly amended, and a new example has been introduced. | |
| Question 8 | New question added on indirect harm related to IVDs. Footnote 19 added. | |
| Question 9 | New question added on expected erroneous results. Footnote 20 added. | |
| Question 11 | New question added on whether to ‘report serious incidents with CE- marked devices used in a clinical investigation or performance study’. Footnote 24 added. | |
| Question 15 | First paragraph, inclusion of following newly added sentence: ‘ (i.e. any natural or legal person acting on behalf of the manufacturer)’. Newly added reference to a field in version 7.3.1 of the MIR: ‘Manufacturer awareness date of reportability’’ | |
| Question 17 | Newly added examples. Third paragraph newly added section, starting with ‘For IVDs, modifications to the clinical management…’ | |
| Question 19 | New question added on preventive and corrective actions. Footnote 33 added. | |
| Question 20 | New text added in last bullet point, starting with ‘For certain serious incidents or FSCAs,…’ | |
| Question 21 | Question revised. Third paragraph newly added on the ‘COMMISSION IMPLEMENTING REGULATION (EU) 2021/2078. Footnote 36 added. | |
| MDCG 2023-3 Rev 2 changes (January 2025) | ||
| Question 1 | Footnote 8 has been amended to align with Regulation (EU) 2024/1860 amending Regulations (EU) 2017/745 and (EU) 2017/746 as regards a gradual roll-out of Eudamed, the obligation to inform in case of interruption or discontinuation of supply, and transitional provisions for certain in vitro diagnostic medical devices. | |
| Question 21 | Reference to ‘Eudamed vigilance (VGL) module’ is amended to ‘Eudamed Post-market surveillance and Vigilance module (VGL module)’. | |
| Footnote 34 | Mention of ’48 working hours’ replaced with ‘allow 48 hours (equivalent to two weekdays)’. | |
Table of Contents
Introduction
This document aims to clarify important terms and concepts that are outlined in Section 2 of Chapter VII of Regulation (EU) 2017/745 on medical devices (MDR) and Regulation (EU) 2017/746 on in vitro diagnostic medical devices (IVDR). Establishing a common understanding of these terms and concepts is necessary for the effective and harmonised implementation of the vigilance requirements under these Regulations. The document is written for competent authorities, economic operators and other relevant parties.
Some of the definitions presented in this document are reintroduced from the Guidelines on a Medical Devices Vigilance System (1) with, where relevant, modifications for alignment with the MDR and IVDR.
Unless quoted directly from the legal text or otherwise specified, the term ‘devices’ will be understood to include medical devices, accessories for medical devices, products listed in Annex XVI of the MDR, in vitro diagnostic medical devices and accessories for in vitro medical devices. Furthermore, references to ‘the Regulations’ should be understood to cover both the MDR and the IVDR.
This document is non-exhaustive and should be read in conjunction with the Regulations, relevant standards (2), and MDCG guidance documents (3).
1. What is the difference between an ‘incident’ and a ‘serious incident’ with a device under the Regulations?
The main difference between an ‘incident’ and a ‘serious incident’ under the Regulations is the severity of the related health or public health outcome (or potential outcome) linked to an issue with a device made available on the market.
The definitions of ‘incident’ and ‘serious incident’ are outlined in the table below.
Medical devices | In vitro diagnostic medical devices | |
|---|---|---|
Regulation | MDR | IVDR |
Incident | An ‘incident’ (Article 2(64) MDR) is any malfunction or deterioration in the characteristics or performance of a device made available on the market, including use-error due to ergonomic features, as well as any inadequacy in the information supplied by the manufacturer (4) and any undesirable side-effect. | An ‘incident’ (Article 2(67) IVDR) is any malfunction or deterioration in the characteristics or performance of a device made available on the market, including use-error due to ergonomic features, as well as any inadequacy in the information supplied by the manufacturer (5) and any harm as a consequence of a medical decision, action taken or not taken on the basis of information or result(s) provided by the device |
Serious Incident | ‘Serious Incident’ (Article 2(65) MDR) means any incident that directly or indirectly led, might have led or might lead to any of the following: (a) the death of a patient, user or other person, | ‘Serious incident’ (Article 2(68) IVDR) means any incident that directly or indirectly led, might have led or might lead to any of the following: (a) the death of a patient, user or other person, |
Incidents that are not classified as serious incidents, are not reportable to competent authorities under Article 87(1) MDR/Article 82(1) IVDR. However, such incidents must be documented and considered in the manufacturer’s quality management system and reported in accordance with requirements outlined in Article 88 MDR/Article 83 IVDR. (6)
‘Serious incidents’ are a subset of incidents. A serious incident is an incident that has in addition, either led to, or has the potential to lead to, any of the health or public health outcomes outlined in Article 2(65)(a) to (c) MDR/Article 2(68)(a) to (c) IVDR.
The manufacturer must report serious incidents in accordance with Article 87(1) – (5) MDR/Article (82) (1) – (5) IVDR to the relevant competent authority. (7, 8)
Reportability under the MDR
If the manufacturer, upon its initial evaluation, determines that an incident is not a serious incident, it must still investigate whether it directly or indirectly might lead to/might have led to, any of the outcomes specified in Article 2(65)(a) – (c) MDR/Article 2(68)(a) – (c) IVDR, if the circumstances were less favourable (for instance, without the performance of an intervention by a third party or if there was exposure of more vulnerable patients to the same situation, etc.).
If the manufacturer cannot exclude that the incident could potentially have led to the outcomes specified in Article 2(65)(a) to (c) MDR/Article 2(68)(a) to (c) IVDR, the incident must be considered serious and reported to the relevant competent authority.
If, after becoming aware of a potentially reportable incident, the manufacturer is uncertain whether the incident is reportable, it must nevertheless submit a report within the timeframe required, in accordance with Article 87(2) – (5) MDR/Article 82 (2) – (5) IVDR. (9)
The flowchart below illustrates the general process for the management of incidents and serious incidents.
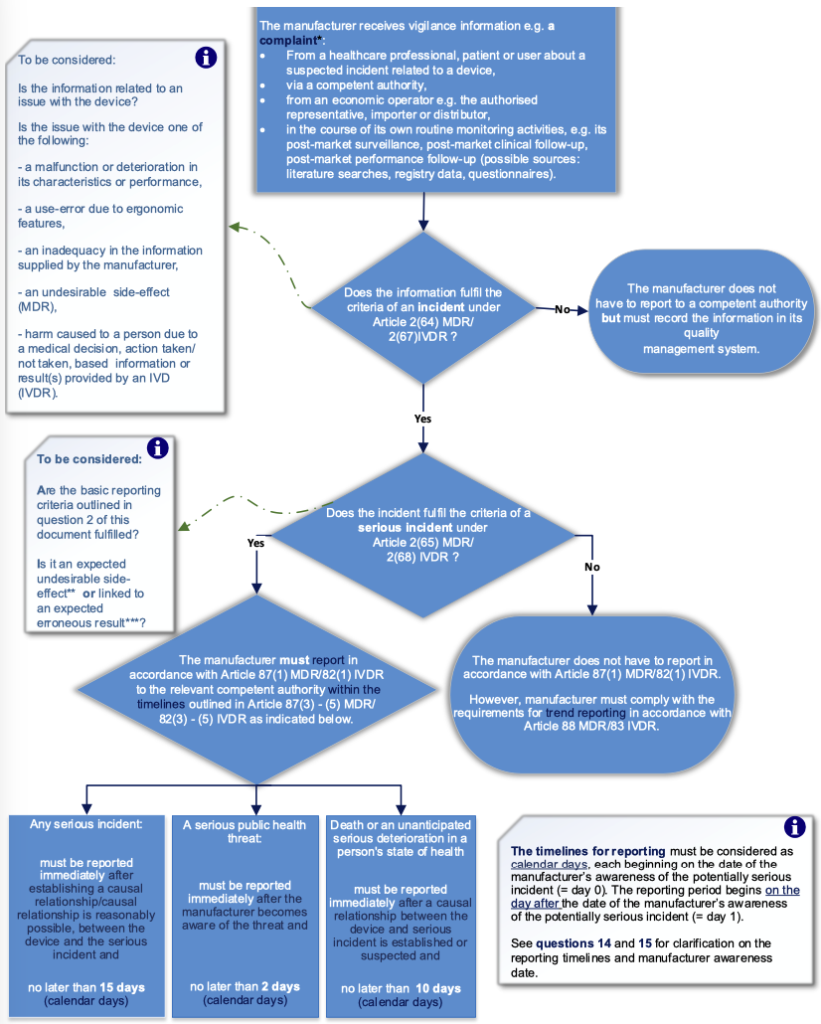
* A ‘complaint’ is defined in EN ISO 13485:2016 and can be described as a written, electronic or oral communication that alleges deficiencies related to the identity, quality, durability, reliability, usability, safety or performance of a device, or related to a service that affects the performance of such devices, irrespective of the source of this information.
** Expected undesirable side-effects must be clearly documented in the product information, quantified in the technical documentation and are subject to trend reporting pursuant to Article 88 MDR (see question 10 for further elaboration on undesirable side-effects).
*** Expected erroneous results must be clearly documented in the product information, quantified in the technical documentation and are subject to trend reporting pursuant to Article 83 IVDR (see question 9 for further elaboration on expected erroneous results).
2. What are the basic reporting criteria for a serious incident?
Any incident which meets all three basic reporting criteria A – C listed below is considered to be a serious incident and must be reported to the relevant competent authority:
A. an incident (Article 2(64) MDR/Article 2(67) IVDR) has occurred, and
B. the incident directly or indirectly led, might have led, or might lead to any of the outcomes of a serious incident (Article 2(65) MDR/Article 2(68) IVDR), and
C. a causal relationship between the serious incident and the manufacturer’s device has been established, is reasonably possible or is suspected.
Elaboration of the reporting criteria A – C is provided below.
Criterion A: occurrence of an incident
Examples of incidents can include the following (non-exhaustive list):
- A device that fails or is losing its ability to achieve its intended purpose (Article 2(12) of the Regulations) when used as instructed in the information supplied by the manufacturer (see question 3 for further clarification of a malfunction or deterioration in the characteristics or performance of the device).
- A deterioration in the characteristics of the device that is related to manufacturing errors, such as sterilisation process failures.
- A use error due to the ergonomic features of the device, such as a use error caused by a mismatch between the user interface (10) and the physical condition of the intended user (see question 6 for further clarification).
- Any inadequacy in the information supplied by the manufacturer, such as insufficient information on how to use, maintain, adjust or calibrate the device, which can lead to a use error.
- Unclear instructions in the labelling or the manufacturer’s instructions for use, such as information that is not written in a manner that is suitable for/readily understood by the intended user.
- Incorrect labelling, such as mislabelling of IVD reagents.
- Undesirable side-effects, such as allergic skin reactions after exposure to nickel or wound therapies (see question 10 for further clarification of undesirable side-effects).
- Harm to a person resulting from a medical decision, action taken, or not taken, based on incorrect information or result(s) provided by the device. For instance, incorrect diagnostic results generated by the device that resulted in delayed patient treatment and caused a deterioration in the patient’s health.
Criterion B: the incident directly or indirectly led, might have led, or might lead to any of the outcomes of a serious incident.
For this criterion to be fulfilled, it is sufficient that an incident associated with the device occurred, and the incident was such that, if it happened again, it might have led or might lead to any of the following outcomes:
- death of a patient, user or other person,
- the temporary or permanent serious deterioration of a patient’s, user’s or other person’s state of health,*
- a serious public health threat.**
* A serious deterioration in the state of health of a patient, user or other person can include:
I. a life-threatening illness or injury,
II. temporary or permanent impairment of a body structure or a body function,
III. a condition necessitating hospitalisation or prolongation of existing hospitalisation,
IV. medical or surgical intervention to prevent I or II, examples of this can be:
▪ professional medical care or additional unplanned medical treatment,
▪ a clinically relevant increase in the duration of a surgical procedure
V. a chronic disease,
VI. foetal distress, foetal death or any congenital abnormality (including congenital physical or mental impairment) or birth defects.
Please note that any indirect harm that may occur as a consequence of a medical decision or action taken/not taken on the basis of information or result(s) provided by a device can also lead to serious incidents, including a serious deterioration in a person’s state of health (see questions 7 and 8 for further clarification of indirect harm).
** A serious public health threat as defined in Article 2(66) MDR/Article 2(69) IVDR should in the context of this document be understood as events that would include:
I. the possibility of multiple deaths occurring at short intervals,
II. events that are of significant and unexpected nature, such that they become alarming as a potential public health hazard.
Examples of serious public health threats, linked to a device, can include the following (non-exhaustive list):
- An IVD test for infectious diseases that fails to perform as intended, potentially affecting a large population group with an infectious disease. For instance, the failure of an IVD test used in a blood bank; this could lead to the widespread distribution of contaminated blood, causing potential exposure to individuals and potentially triggering an outbreak of an infectious disease.
- High risk of disease progression due to exposure to carcinogenic, mutagenic or reprotoxic (CMR) chemicals linked to the use of a device, which affects a significant portion of the population, a specific patient population (e.g., diabetics, cardiac patients), or a vulnerable population (e.g., children, pregnant women).
- Widespread distribution of falsified or incorrectly labelled devices, leading to multiple serious incidents (e.g. distribution of non-sterile devices labelled as sterile).
- Cyberattack related to life supporting or life-saving devices.
Identifying these threats will depend on manufacturers’ trending of multiple events of the same or similar nature, root causes, exposure routes etc., and may require information concerning multiple devices from multiple manufacturers.
Criterion C: causal relationship between the serious incident and the manufacturer's device has been established, is reasonably possible or suspected.
The manufacturer must investigate whether there is a causal relationship between the serious incident and their device, or if such a relationship is reasonably possible, i.e., the device cannot reasonably be excluded as a contributory cause of the serious incident.
In assessing the potential link between their device and the serious incident, the manufacturer should take account of factors such as:
- clinical or medical plausibility,
- the opinion of healthcare professionals,
- the results of the manufacturer’s own preliminary assessment,
- known information provided in the technical documentation and evidence of previous similar serious incidents,
- other relevant evidence held by the manufacturer.
Establishing or identifying the link between the manufacturer’s device and the serious incident may be difficult, especially when there are multiple devices and drugs involved. In complex situations, it should be assumed that the device may have, or potentially could have, contributed to the serious incident, and the manufacturer should therefore be cautious in its evaluation and conclusions. In case of doubt, the manufacturer must nevertheless submit the report referred to in Article 87(1) MDR/Article 82(1) IVDR. (11)
3. With reference to Article 2(64) MDR/Article 2(67) IVDR, what is meant by a ‘malfunction or deterioration in the characteristics or performance of a device’?
A ‘malfunction (12) or deterioration (13) in the characteristics or performance of a device’ can be described as a situation where a device fails to achieve, or is unable to uphold, the performance (Article 2(22) MDR) stated by the manufacturer when used in accordance with the information supplied with the device. For IVDs, the performance of a device (Article 2(39) IVDR) consists of the analytical and, where applicable, the clinical performance supporting its intended purpose. (14) This device performance must be substantiated by the manufacturer in the technical documentation.
Examples of device malfunctions may include the following (non-exhaustive list):
- A device that, due to a software error, fails to make a correct assessment and provides an incorrect treatment to the patient.
- An electrical short circuit causing the device to catch fire or stop working.
- Premature battery depletion, such as a malfunction resulting in high current drain depleting the device battery faster than indicated in the instructions for use.
- A device that breaks during use even though it was used/handled in accordance with the instructions for use.
Examples of deteriorations in the characteristics or performance of a device may include the following (non-exhaustive list):
- A gradual occlusion of fluid or gas path, change in resistance to flow or electrical conductivity of a device as a result of ageing or repeated use, which negatively affects the performance or characteristics of the device. se,
- A sensor drift caused by physical changes, such as a gradual decrease in accuracy of a sensor caused by airborne pollutants (dust, chemicals, vapor and other contaminants).
- UV degradation of the device such as cracking or disintegration of device materials due to ultraviolet radiation, such as sunlight exposure, which limits the performance of the device.
- Elasticity changes (increase or decrease), such as in compression stockings that, due to an increase in elasticity, are no longer suitable for their intended use.
- Failure of a device component or other types of significant loss of electrical, material or mechanical integrity of the device due to wear or fatigue.
IVD-specific examples of device malfunctions or deterioration in characteristics or performance may include the following (non-exhaustive list):
- Blood glucose test strips that provide incorrect readings, confirmed by an alternative diagnostic testing method, which lead to the incorrect administration of diabetes medication.
- A device software that incorrectly links analysis results to the wrong patients, resulting in incorrect diagnoses and potentially leading to incorrect patient treatments.
- Essential functionality for an automatic analyser fails, causing e.g. incorrect sample mixing, temperature, or pH level for the sample analysis. This can potentially lead to inaccurate analysis results (false positive or false negative), which in turn may cause incorrect patient diagnosis and incorrect treatment.
- Contamination of culture medium for propagating specific microorganisms, leading to incorrect diagnosis (false positive results) and potentially incorrect or unnecessary treatment of patients.
- Any changes in the limit of detection, limit of quantitation, diagnostic specificity, or analytical sensitivity over time, leading to an increase in the frequency of false negatives and/or false positives, which may eventually lead to a change in the benefit-risk ratio.
- Device stability that is significantly decreased before the expiry date, such that the number or ratio of invalid results increases above the specified limit.
The manufacturer should always conduct the necessary investigations, including a root cause analysis, when a device, used in accordance with information supplied by the manufacturer, fails to achieve or uphold its intended performance.
4. Who is considered as the ‘user’ of a device?
For the purpose of this document, the ‘user’ (Article 2(37) MDR/Article 2(30) IVDR) is any healthcare institution, healthcare professional or layperson (e.g., caregiver, patient) who uses the device, or persons responsible for installing or maintaining the device.
5. What is a ‘use error’ in comparison to ‘abnormal use’?
A ‘use error’ is when the user’s action, or lack thereof, while using the device, leads to a different result or outcome than that expected by the user or intended by the manufacturer. (15)
Use errors can be caused by a user’s failure to pay attention, memory lapses, mistakes during device use, or a lack of understanding or knowledge in relation to device use. Such use errors do not fall within the definition of an incident. However, use errors that are caused by the ergonomic features of a device qualify as incidents. When these incidents, fulfil the criteria of serious incidents, they must be reported by the manufacturer to the competent authorities under Article 87(1) MDR/Article 82(1) IVDR (see question 6 for elaboration).
Use errors must be documented and handled within the manufacturer’s quality management system.
‘Abnormal use’ is the deliberate violation of the intended use of a device. It is a deliberate act or omission of an act by the user that is counter to or violates the normal use of a device and is beyond any further reasonable means of interface-related risk control measures by the manufacturer.
An example of abnormal use may include off-label use of a device, such as a healthcare professional who, based on a medical decision, uses a device for an indication different from that specified in the manufacturer’s instructions for use.
Any information related to abnormal use of a device that reaches the manufacturer, including from healthcare professionals or through other post-market surveillance activities, must be documented and managed within its quality management system. (16, 17)
6. What is a ‘use-error due to ergonomic features’ as mentioned in the Regulations?
‘Use-error due to ergonomic features’ can be described as use errors caused by the design and physical configuration of the device, including the features with which the intended user interacts.
The ergonomic features of the device include components such as measurement and monitoring features, display scales, alarms, software menus, and any other factors related to the user interface.
Use errors due to ergonomic features are not caused by factors such as user negligence but are the result of an ergonomic design that makes it difficult for the intended user to operate the device correctly (i.e., as intended by the manufacturer), thereby compromising its safe use. Such use errors may arise from a mismatch between the device features (including the information provided in the instructions for use) and factors such as the user profile (18) and/or the environment in which the device is intended to be used.
It should be noted that, in some cases, use errors caused by ergonomic features may not be immediately identified and can lead to serious outcomes due to the unintentional nature of the error and the potential for the user to be unaware of its occurrence. This note is especially important for devices where the patient is responsible for installing or adjusting their treatment, or devices used in therapeutic applications, e.g., drug-delivery devices or devices with a diagnostic or measuring function.
Use errors due to ergonomic features must in the case of a serious incident, be reported in accordance with Article 87(1) – (5) MDR/Article 82(1) – (5) IVDR, or in case of incidents, reported in accordance with Article 88 MDR/Article 83 IVDR.
7. How can incidents involving a device indirectly lead to a serious deterioration in a person’s state of health?
Some devices, due to their intended use, may not lead to direct (or immediate) harm or a deterioration of a person’s health but may instead cause indirect harm.
In most cases, harm or a deterioration of health will be indirect if arising from an incident linked to a medical decision, actions taken, or lack thereof, which are based on incorrect information, or results provided by a device.
Potential sources of indirect harm linked to a medical device or IVD may include (non-exhaustive list):
- a misdiagnosis,
- a delayed diagnosis,
- delayed treatment,
- inappropriate treatment,
- absence of treatment,
- transfusion of inappropriate materials.
An example of indirect harm is a device that provides imprecise readings, which results in an incorrect medical decision and, consequently, an incorrect medical treatment. For instance, a blood pressure monitoring device that fails to provide accurate measurements. Based on these incorrect measurements, the user of the medical device administers an incorrect dosage of medication, which leads to a serious deterioration in this person’s health and requires further medical treatment.
Indirect harm, due to an incident that meets or has the potential to meet the outcomes of a serious incident, must be reported in accordance with Article 87(1) – (5) MDR/Article 82 (1) – (5) IVDR.
8. What is considered ‘any harm as a consequence of a medical decision, action taken or not taken on the basis of information or result(s) provided by the device’ as outlined in Article 2(67) IVDR? How is it reported within the IVDR vigilance system?
This should be understood to include any harm to a person’s health, which is not physically and directly caused by an IVD but is a consequence of a medical decision (19), action taken or not taken, based on incorrect information or results obtained with the IVD.
In the majority of cases, IVDs will, due to their use on specimens, rather than directly on the human body, not lead to direct harm of a person’s health. These devices are more likely to lead to indirect patient harm, which is caused by a medical decision, action taken or lack thereof based on incorrect information or result(s) provided by the IVD.
An example of indirect harm linked to these devices is an IVD that provides results with insufficient accuracy, which results in an incorrect medical decision by the healthcare professional responsible for patient management. This incorrect decision leads to an inappropriate medical treatment of the patient, which leads to a serious deterioration in this person’s health.
Any harm that is a consequence of a medical decision, action taken or not taken based on incorrect information or result(s) provided by an IVD, qualifies as an incident under Article 2(67) IVDR. Such incidents, which do not meet the criteria of a serious incident, must be reported in accordance with the requirements for trend reporting under Article 83 IVDR.
9. What is an ‘expected erroneous result’ and how is it reported within the IVDR vigilance system?
An ‘erroneous result’ should be understood to include any incorrect (i.e. inadequate, inaccurate or imprecise) result or information provided by an IVD.
Expected erroneous results are established in comparison to the stated performance of the device as referred to in points (a) and (b) of Section 9.1 of Annex I of the IVDR and must be clearly documented and quantified in the product information and the technical documentation.
They should also be acceptable when weighed against the evaluated benefits to the patients and/or user arising from the achieved performance of the device during normal conditions of use (Section 8 of Annex I IVDR). (20) Expected erroneous results should be reported in accordance with the requirements for trend reporting pursuant to Article 83 IVDR.
Erroneous results that are not documented and quantified in the product information and the technical documentation and hence fall outside the declared performance of the IVD, are to be handled like incidents. If such incidents qualify as serious incidents within the meaning of Article 2(68) IVDR, they should be reported in accordance with Article 82(1) IVDR as individual serious incident reports (i.e. as individual Manufacturer Incident Reports (MIRs)).
If the manufacturer cannot demonstrate that a potentially serious incident is an expected erroneous result within the deadlines set out in Article 82(3) – (5) IVDR, it should be considered a serious incident, and a MIR should be submitted within the defined timelines.
10. What is an ‘undesirable side-effect’ and how is it reported within the MDR vigilance system?
An ‘undesirable side-effect’ under the MDR should be understood as any unintended and unwanted medical manifestation in the human body, as a consequence of the normal use of a medical device. (21) Undesirable side-effects are not the result of a malfunction, deterioration in the characteristics or performance of the medical device, or an inadequacy in the information supplied by the manufacturer.
An unsuccessful treatment (or treatment failure) should not be considered an undesirable side effect.
For the purpose of this guidance, undesirable side-effects can be expected or unexpected and are considered as incidents under the MDR (Article 2(64) MDR). (22)
Expected undesirable side-effects must be clearly documented in the product information and quantified in the manufacturer’s technical documentation. They must also be acceptable when weighed against the evaluated benefits to the patient and/or user arising from the achieved performance of the medical device during normal conditions of use (Section 8 of Annex I MDR). (23)
Expected undesirable side-effects must be reported in accordance with the requirements for trend reporting pursuant to Article 88 MDR.
If the manufacturer cannot demonstrate that a potentially serious incident is an expected undesirable side-effect within the deadlines set out in Article 87(3) – (5) MDR, they must submit a MIR within the timelines.
Unexpected undesirable side-effects are not considered in the manufacturer’s risk analysis, quantified in the manufacturer’s technical documentation or documented in the product information. If they occur, they are to be handled like all incidents. That means that, if they qualify as serious incidents within the meaning of Article 2(65) MDR, such side-effects must be reported in accordance with Article 87(1) MDR as individual serious incident reports (i.e. individual MIRs).
11. Is it required to report serious incidents with CE- marked devices used in a clinical investigation or performance study?
Yes. CE-marked devices used in clinical investigations or performance studies are subject to the vigilance reporting requirements. Serious incidents related to such devices must be reported by the manufacturer in accordance with Article 87(1) MDR/Article 82(1) IVDR. (24)
The vigilance reporting provisions apply in the following cases:
- Serious incidents with any CE-marked device used within the intended purpose covered by the CE-marking, in a clinical investigation or performance study.
- Serious incidents with any CE-marked device used within the intended purpose covered by the CE-marking, in a post-market clinical follow-up (PMCF) investigation or post-market performance follow-up (PMPF) study.
12. Articles 87(5) MDR and 82(5) IVDR outline the timelines for manufacturers to report an unanticipated serious deterioration in a person's state of health. When is a serious deterioration in a person's state of health considered ‘unanticipated’?
A serious deterioration in a person’s state of health, is regarded as ‘unanticipated’ if the condition leading to the deterioration was not considered in the manufacturer’s risk assessment.
A serious deterioration in a person’s state of health is regarded as anticipated if it was considered in the manufacturer’s risk analysis and documented in the risk management report.
For a serious deterioration in the state of health, the manufacturer must ensure there is:
- documented evidence that a risk management method was used to eliminate or reduce the risk related to these events as far as possible, or
- the risk is included in the information supplied by the manufacturer to the user, e.g. in the instructions for use.
13. With reference to the timelines for the vigilance reporting requirements outlined in the Regulations, what is meant by ‘immediately’ and ‘without undue delay’?
For the purpose of this document, ‘immediately’ and ‘without undue delay’ should both be understood as meaning without any delay that is intentionally or negligently caused by the manufacturer.
As a general rule, the report outlined in Article 87(1) MDR/Article 82(1) IVDR must be provided without any delay and no later than the reporting timelines outlined in Article 87(3)-(5) MDR/Article
82(3) – (5) IVDR. To ensure timely reporting, the manufacturer can submit an initial MIR within the specified timelines, which can then be followed by a follow-up report (Article 87(6) MDR)/ Article 82(6) IVDR).
Question 14 of this document provides further clarification on how to apply the reporting timelines.
14. How to apply the reporting timelines defined by Article 87(3) to (5) MDR/Article 82 (3) to (5) IVDR?
In accordance with the Regulations, the reporting referred to in Article 87(1) MDR/Article 82(1) IVDR must take account of the severity of the serious incident.
The timelines for reporting serious incidents must be considered as calendar days, meaning the reporting periods include weekdays, public holidays, Saturdays and Sundays (25).
As a general rule, the reporting period begins on the day after the awareness date of a potentially serious incident (26) at 00:00:01 AM (27). The awareness date (day=0) refers to the date when the manufacturer is first made aware or receives information of the occurrence of the (potentially) serious incident and not after it has conducted its investigation. See also question 15 for elaboration on the ‘manufacturer awareness date’.
The reporting timelines outlined in the Regulations are the following:
- any serious incident that did not involve a death or an unanticipated serious deterioration in a person’s state of health must be reported immediately, and no later than 15 days after the awareness date of the serious incident (Article 87(3) MDR/Article 82(3) IVDR),
- a serious public health threat must be reported immediately, and not later than 2 days after the manufacturer becomes aware of that threat (Article 87(4) MDR/Article 82(4) IVDR),
- death or an unanticipated serious deterioration in a person’s state of health must be reported immediately and no later than 10 days after the awareness date of the serious incident (Article 87(5) MDR/Article 82(5) IVDR).
In the exceptional cases where a manufacturer initially determines that an incident does not meet the reporting requirements of a serious incident but later obtains new information that impacts or changes the initial reportability assessment, resulting in the incident meeting the reporting requirements of a serious incident, the period of reporting begins on the date the manufacturer received the information that determined that the incident is reportable.
In both situations i.e., the general rule and the exceptional case, the period ends on the 15th, 2nd or 10th day thereafter (more specifically at 11:59:59 PM (28)). However, if this (last) day is a public holiday, Saturday or Sunday the deadline is moved to the following working day automatically (29).
Nevertheless, in line with Article 87(3) – (5) MDR/Article 82(3) – (5) IVDR, outlining the requirement to report ’immediately’ but not later than the period provided for in those provisions, it is highly recommended for the manufacturer to report immediately or as early as possible in time.
A delay in submitting an initial report e.g. due to incomplete information provided by the healthcare facility, end user or other relevant parties, is not deemed justified. As outlined in Article 87(6) MDR/Article 82(6) IVDR, the manufacturer can submit an initial MIR followed up by a follow-up report providing additional information related to the (potentially) serious incident and progress on the incident investigation(s). Any report must not be unduly delayed because of incomplete information.
The example below demonstrates the timelines for the exceptional case described above, in which the manufacturer is first made aware of an incident and concludes that it does not meet the reporting requirements of a serious incident, but later obtains new information that impacts or changes the manufacturer’s previous determination regarding the need to report.
Example
A manufacturer receives a complaint on 1st June 2022. The manufacturer determines that the criteria for a serious incident were not met and, therefore, does not submit a MIR to the relevant competent authority.
The manufacturer subsequently receives additional information on 1st July 2022. Upon review of this information, the manufacturer determines that the complaint is a serious incident and reportable under Article 87(3) MDR/82(3) IVDR. The manufacturer must submit a MIR at the latest by 16th of July 2022.
Variation of the above example:
On 2nd July 2022, the manufacturer was made aware that the patient died on 2nd July 2022. Since the consequence of the serious incident is now a patient death, a report must be submitted no later than 10 days after awareness date of the serious incident. Therefore, a MIR must be submitted at the latest by 12th July 2022. In conclusion, it is the earliest date of reporting which should be considered.
15. With reference to the reporting timelines of a serious incident pursuant to Article 87 MDR/Article 82 IVDR, what is considered as the manufacturer awareness date?
For the purpose of this document, the ‘manufacturer awareness date’ of the incident is the date when the first employee or representative of the manufacturer’s organisation (i.e. any natural or legal person acting on behalf of the manufacturer) receives information regarding the potentially serious incident. If the handling of this information is performed by the authorised representative or if the manufacturer has outsourced its complaint and incident handling activities to another natural or legal person (e.g. a subcontractor), then reference to ’manufacturer’s organisation’ in the context of the awareness date will also apply to that organisation.
In the exceptional cases where a manufacturer initially determines that an incident does not meet the reporting requirements for a serious incident but later obtains new information that changes the manufacturer’s previous determination regarding the need to report, the serious incident must be reported to the relevant competent authority and indicated in the MIR. In the MIR, the manufacturer should provide the relevant dates in the following two fields (30):
- 1.2.c ‘Manufacturer awareness date of the incident’ (In this field, the manufacturer should insert the initial awareness date of the incident).
- 1.2.d ‘Manufacturer awareness date of reportability’ (In this field, the manufacturer should insert the date on which it received the information that determined that the incident is reportable).
Within the general comments in Section 5 of the MIR, the manufacturer should explain the difference between the two dates.
It should be noted that if the manufacturer is uncertain about whether the incident qualifies as a serious incident, it must nevertheless submit a MIR within the reporting timelines.
An example is provided below to demonstrate how to correctly apply the awareness date in the MIR in the exceptional cases described above. In this example, a MIR was initially not submitted to the relevant competent authority as the manufacturer determined that the requirements for reporting a serious incident were not met.
Example
A manufacturer receives a complaint on 1st July 2022. The manufacturer determines that the requirements for a serious incident are not fulfilled and, therefore, does not submit a MIR to the relevant competent authority.
The manufacturer subsequently receives additional information on 1st August 2022 and upon reviewing this information, the manufacturer determines that the complaint qualifies as a serious incident.
The manufacturer should then submit a MIR within timelines outlined under the applicable sections of the MDR/IVDR, i.e. immediately and not later than 15, 2 and 10 days (Article 87(3) to (5) MDR/Article 82(3) to (5) IVDR), from (the day after) 1st August 2022. In the MIR, the manufacturer should insert the dates as follows:
- 1.2.c Manufacturer awareness date of the incident: 1st July 2022
- 1.2.d Manufacturer awareness date of reportability: 1st August 2022
16. Why does the MIR have a report type named ‘Final (Non-reportable incident)’ and when can it be used?
The report type ‘Final, (Non-reportable incident)’ is included in the MIR for cases where the manufacturer has submitted a MIR to the relevant competent authority but establishes through its investigation that the criteria for a serious incident were not met.
The report type ‘Final (Non-reportable incident)’, should be used in the following cases:
- According to Article 87(7) MDR/Article 82(7) IVDR, in case of uncertainty, the manufacturer is obliged to report a potentially serious incident within the timeframe outlined in those articles. However, during this timeframe, the manufacturer may be unable to establish whether the reporting requirements for a serious incident are fulfilled. After submitting a MIR to the relevant competent authority, within the timeframe, further root cause investigation of the device in question could clarify that the criteria for a serious incident were not fulfilled, thus, making the case not reportable.
- Cases where the manufacturer’s analysis of additional information, received after a MIR has been submitted to the relevant competent authority reveals that the reporting requirements of Article 87(1) MDR/Article 82(1) IVDR were not met.
In the abovementioned cases, the manufacturer can select the box ‘Final (Non-reportable incident)’ under section 1.2 of the MIR and provide a rationale for its conclusion in section 4.2.
It should be noted that the report type ‘Final (Non-reportable incident)’ may also be used for cases where the manufacturer has received a report of a potentially serious incident from the competent authorities (Article 87(11) MDR/Article 82(11) IVDR) but establishes within the specified timelines that the requirements for a serious incident are not fulfilled. In such cases, the manufacturer can submit a final MIR, selecting the report type ‘Final (Non-reportable incident)’ and provide a rationale for this conclusion in section 4.2.
If the manufacturer has not finalised its root cause analysis or established the cause and/or contributing factors, the case cannot be considered to qualify as non-reportable. In such instances, a MIR with the report type ‘Final (Non-reportable) incident’ should not be submitted to the competent authority.
17. What is a ‘field safety corrective action’?
A ‘field safety corrective action (FSCA)’ (Article 2(68) MDR/Article 2(71) IVDR) is a corrective action taken by a manufacturer for technical or medical reasons to either prevent or reduce the risk of a serious incident, which is associated with a device that is made available on the market.
Examples of FSCAs are provided below (non-exhaustive list):
- The return of a device to the manufacturer/supplier or a recall31 e.g. due to incorrect labelling of reaction tubes.
- A device exchange.
- A device modification.
- Retrofit by purchaser of manufacturer’s modification or design change.
- A device destruction.
- Advice given by manufacturer regarding the use of the device, such as additional information on maintenance, increased frequency of calibration, cleaning instructions, and training.
- Recommended inspections/examination by device user (e.g. regular professional checks of proper functioning in a testing setting).
- Changes of software/firmware in the device that are related to a safety issue (e.g. version update or rollback to an earlier version).
- Correction of information provided on the labelling, such as corrections to the originally validated and stated shelf life of the device.
- Changes to the packaging design of the device that will correct safety-related issues.
- Instructions provided by the manufacturer concerning modifications to the clinical management of patients /samples, such as a recall of patients or patient samples for retesting, or reviews of previous test results.
Advice given by a manufacturer may include modifications to the clinical management of patients to address a risk of death or a serious deterioration in the state of health specifically related to the characteristics of a device. An example of this is cases involving implantable devices, where it is often clinically unjustifiable to explant the device. Therefore, special patient follow-up or treatment, irrespective of whether any affected un-implanted devices remain available for return, constitutes a measure to be included in a FSCA. For IVDs, modifications to the clinical management of patients to address a specific risk may include cases where it is clinically unjustifiable to obtain new patient samples, retest, or reanalyse existing ones. In such cases, instructions from the manufacturer on additional patient follow-up or monitoring may also constitute measures to be included in a FSCA.
A FSCA must be communicated/transmitted without undue delay for the attention of users or customers of the device in question through a field safety notice (FSN) (Article 2(69) MDR/Article 2 (72) IVDR) sent by the manufacturer.
Unless duly justified by the situation of the individual Member State (e.g. a translation error in the instructions for use that appears only in certain languages and therefore affects only specific countries), the content of the FSN must be consistent in all Member States. The requirements for the content of the FSN are outlined in paragraph 2 of Article 89(8) MDR/Article 84(8) IVDR.
Example of a FSCA conducted by a manufacturer:
As part of its post-market surveillance activities, a manufacturer identifies a systematic device malfunction.
If the device malfunction affects devices that are available on the market and these devices have led or potentially could lead to a serious incident, the manufacturer must initiate a FSCA to either prevent or reduce the risk of such incidents. The FSCA implemented by the manufacturer may include permanent or temporary changes to the device’s labelling or instructions for use or a recall of all affected devices that are available on the market.
The manufacturer must inform the relevant competent authority(ies) in the Member State(s) where the FSCA has been or will be undertaken, as well as the Member State where it has its place of business, without delay. Additionally, the manufacturer must ensure that information about the FSCA is communicated without delay to the affected users though a FSN.
18. With reference to Article 87(1)(b) MDR/Article 82(1)(b) IVDR, what is meant with ‘…including any field safety corrective action undertaken in a third country…’?
For FSCAs undertaken in a third country where the device is also legally made available on the Union market, (32) all relevant competent authorities must be notified unless the reason for the FSCA is limited to devices made available in the third country.
Example
An example of such a FSCA would be where a recall of a device has taken place in a third country due to a malfunction with certain lots. If the lots affected by this recall have also been made available on the Union market, all relevant competent authorities must be notified of the FSCA.
19. Should manufacturers inform competent authorities of preventive or corrective actions in accordance with 83(4) MDR and 78(4) IVDR?
If a manufacturer identifies a need for a safety-related preventive or corrective action linked to a serious incident or a field safety corrective action (as referred to in Article 83(4) MDR/78(4) IVDR, second sentence), it must report this information to the competent authorities.
This information must be provided using the relevant vigilance report(s), i.e., the MIR or field safety corrective action form. In these reports, the manufacturer should provide a relevant description of the safety-related preventive or corrective action and the associated reference or case number.
Preventive or corrective actions which are covered by Article 83(4) MDR/Article 78 (4) IVDR, first sentence, can be made available to the competent authorities either through the periodic safety update report (PSUR) or post-market surveillance report. Further relevant information can be found in Chapter 2.1.2 of the PSUR guidance, MDCG 2022-21 (33).
20. Within the scope of Article 89 MDR/Article 84 IVDR on analysis of serious incidents and field safety corrective actions, who is the ‘evaluating competent authority’?
The ‘evaluating competent authority’ is the national competent authority of the Member State responsible for assessing the risks arising from reported serious incidents that occur within its territory, and/or for evaluating the adequacy of FSCAs envisaged or undertaken, by the manufacturer within its territory, (Article 89(2) and (3) MDR/Article 84(2) and (3) IVDR).
In the scenarios provided below, the evaluating competent authority is:
- For serious incidents: the competent authority of the Member State in which the serious incident occurred.
- For FSCA(s): the competent authority(ies) of the Member State(s) in which the FSCA is being or is to be undertaken, e.g. Member States in which the devices affected by the FSCA are made available.
The competent authority in the Member State where the manufacturer or its authorised representative has its registered place of business must always be informed of the FSCA, even if it is not amongst the Member States in which the FSCA is being or is to be undertaken.
In the context of commenting on the content of the draft FSN as outlined in paragraph 1 of Article 89(8) MDR/Article 84(8) IVDR, manufacturers must, except in cases of urgency, submit the draft FSN, to the evaluating competent authority to allow it to review and make comments. (34)
In the cases referred to in 89(9) MDR/Article 84(9) IVDR, the draft FSN must be submitted to the designated coordinating competent authority. However, the final FSN must be transmitted to all evaluating competent authorities. - In the cases referred to in Article 89(9) MDR/Article 84(9) IVDR, the evaluating competent authorities are the authorities that participate in the coordinated assessment procedure. For certain serious incidents or FSCAs, which are considered to be of concern, the evaluating competent authorities, may participate in a procedure aimed at coordinating their assessments under the direction of a designated coordinating authority (Article 89(3) MDR/Article 84(3) IVDR). Once designated, the coordinating competent authority must inform the manufacturer, along with the other competent authorities and the Commission, through EUDAMED or alternative means that it has assumed this role. (35)
21. When can Eudamed be used for vigilance reporting?
The European database on medical devices (Eudamed) is described in Article 33 MDR/Article 30 IVDR. The specific requirements related to the electronic system on vigilance (referred to hereafter as the ‘Eudamed Post-market surveillance and Vigilance module (VGL module)’ or the ‘vigilance module’) are outlined in Article 92 MDR/Article 87 IVDR.
In line with Article 34(2) and (3) MDR, as amended by Regulation 2024/1860, the Eudamed vigilance module will be declared functional once it is audited and a Commission notice confirming the functionality of the module is published in the Official Journal of the European Union (OJEU). The obligation and requirements for the Eudamed VGL module will become applicable six months after the publication of this notice in the OJEU. (36)
Until the Eudamed VGL module becomes mandatory for use, competent authorities and economic operators must continue to use the national reporting processes as explained in MDCG 2021-1 Rev. 1 and MDCG 2022-12 for compliance with the vigilance provisions outlined in the Regulations.
After the mandatory use of the Eudamed VGL module, in case of technical unavailability or malfunction of the system, the relevant parties should follow the instructions on alternative mechanisms to exchange data, as described in Article 8 of the Commission Implementing Regulation (EU) 2021/2078 of 26 November 2021 laying down rules for the application of Regulation (EU) 2017/745 of the European Parliament and of the Council as regards the European Database on Medical Devices (Eudamed). (37)
22. In accordance with Articles 10, 13 and 14 of the Regulations, manufacturers, importers and distributors are required to inform competent authorities of devices that present or are considered to present a serious risk. What is meant by a ‘serious risk’?
For the purpose of this document, a ‘serious risk’ is defined as a situation where use a device is likely to cause serious harm to patients, users or the public (38). A serious risk may include situations where the effects of the risk are not immediate.
For cases involving a device that presents a serious risk, the manufacturer, importer or distributor must immediately inform the competent authorities of the Member States in which they made the device available and, where applicable, the notified body that issued a certificate for the device in accordance with Article 56 MDR/Article 51 IVDR. (39)
23. What is a ‘Periodic Summary Report’?
A ‘Periodic Summary Report’ (PSR) is an alternative reporting regime by which the manufacturer, in agreement with the respective national competent authority that is coordinating the periodic summary reporting (and in consultation with the competent authorities referred to in Article 92(8) (a) MDR/Article 87(8)(a) IVDR), can report similar serious incidents with the same device or device type in a consolidated way. Criteria for periodic summary reporting include situations where; the root cause has been identified, a FSCA has been implemented or where the serious incidents are common and well documented.
The requirements for periodic summary reporting are outlined in Article 87(9) MDR/Article 82(9) IVDR.
24. What are the criteria for a ‘common and well documented’ serious incident?
A ‘common and well documented serious incident’ as referenced in Article 87(9) MDR/Article 82(9) IVDR, must be clearly identified in the manufacturer’s risk analysis and should have led to incident reports, which have been assessed by the manufacturer and the relevant competent authority. The serious incident and the root cause should be clinically well-known (i.e. a certain qualitative or quantitative predictability is established) by the manufacturer.
Footnotes
(1): Guidelines on a Medical Devices Vigilance System, MEDDEV 2/12-1 rev. 8, January 2013. Please note that the MEDDEV 2.12/1 rev. 8, January 2013 was in operation under the Directives (Directive 93/42/EEC (MDD), Directive 90/385/EEC (AIMDD)) and Directive 98/79/EC (IVDD)). It is not aligned with the requirements of the Regulations and is considered. obsolete.
(2): For definition of a ‘standard’ please refer to Article 2(1) Regulation (EU) No 1025/2012 of the European Parliament and of the Council of 25 October 2012 on European standardisation. A summary list of titles and references of harmonised standards can be found on the European Commission Medical Devices website: Summary list of titles and references of harmonised standards
(3): All MDCG Guidance documents can be found on the European Commission Medical Devices website: Guidance documents.
(4): The general requirements for the ‘information supplied by the manufacturer’ for medical devices are outlined in Section 23 of Annex I of the MDR and further clarification is provided in EN ISO 15223-1:2021. For in vitro diagnostic medical devices, this information is outlined in Section 20 of Annex I of the IVDR, and further clarification is provided in ISO 18113-1:2022. The information supplied by the manufacturer should be regarded as part of the medical device or accessory. For the purpose of this document, this information includes the label (packaging or marking), instructions for use, technical description, installation manual, quick reference guide, training material, any promotional material, sales material, statements by the manufacturer and other information accompanying the device.
(5): See footnote 4.
(6): Trend reports (Article 88 MDR/Article 83 IVDR) should be reported to the competent authority(ies) of the Member State(s) in which the incidents occurred.
(7): In this context, the relevant competent authority is the competent authority of the Member State in which the serious incident occurred. Serious incidents are to be reported to the competent authorities by means of the Manufacturer Incident Report (MIR), which became applicable on 1st January 2020. The MIR and the MIR-help text can be found on the European Commission Medical Devices website: Guidance documents.
(8): Once the Eudamed Post-market surveillance and Vigilance module becomes mandatory to use, notified body(ies) will be able to see the vigilance reports in which they are referenced. In the interim, manufacturers and notified bodies are advised to agree on how that information is provided to the notified body (that issued the certificate for the device in question) and may continue using the same procedures as those under the Directives (MDD, AIMDD and IVDR).
(9): Article 87(7) MDR/ Article 82(7) IVDR.
(10): For the purpose of this document, and in line with EN 62366-1:2015, the ‘user interface’ covers all the elements of the medical device with which the user interacts. This includes the physical aspects of the device, as well as visual, auditory, and tactile displays, and is not limited to the software interface. Additionally, for the purpose of this document, the information supplied by the manufacturer, such as the accompanying information, is considered part of the device and its user interface.
(11): As outlined in Article 87(7) MDR/ Article 82(7) IVDR.
(12): See also the following definition of a ‘malfunction’ provided in EN ISO 14155:2020: ‘A malfunction is a failure of a device to perform in accordance with its intended purpose when used in accordance with the manufacturer’s instructions for use or to meet its performance specifications’.
(13): For the purpose of this document, the following definition of deterioration was taken into account: ‘the action or process of a device becoming impaired or inferior in quality, function or condition’ (Merriam-Webster.com Dictionary, Merriam-Webster, Link to definitions).
(14): ‘Analytical performance’ and ‘clinical performance’ are defined in Article 2(40) IVDR and Article 2(41) IVDR, respectively
(15): For the purpose of this document and in line with the information provided in EN ISO 14971:2019, use errors should be understood to include the inability of the user to complete the task when using the device. Furthermore, the occurrence of an unexpected physiological response of the patient when using the device should not, by itself, be considered a use error as well as a malfunction of a device that leads to an unexpected result.
(16): Based on data, (e.g., from complaints) related to abnormal use, manufacturers might be able to identify possible systematic misuse or off-label use of their device and verify that the device’s intended purpose is appropriate and, where relevant, identify the need for initiating any field safety corrective action(s).
(17): Abnormal use should be distinguished from reasonably foreseeable misuse, which is defined as the use of a product or system in a manner not intended by the manufacturer, but which can result from readily predictable human behavior (ISO 14971:2019(EN)). Reasonably foreseeable misuse must be documented within the manufacturer’s quality management system.
(18): For the purpose of this document, and also in line with information provided in EN 62366-1:2015/AMD1, the user profile includes ‘the mental, physical or demographic traits of the intended user group, as well as any special characteristics, such as occupational skills, job requirements and working conditions of the user, which can have a bearing on design decisions related to the device’.
(19): For some IVDs (e.g., self-testing devices), the medical decision is made by the user of the device, who is also the patient.
(20): In this context, reference to ‘patient’ should be understood as the individual patient i.e., acceptable in terms of the individual patient benefit.
(21): It should be noted that the terms ‘undesirable side-effects’ and ‘side-effects’ are used synonymously in the MDR.
(22): In addition to footnote 17, note also for reporting purposes that the terms: ‘identified side-effects’, ‘expected side-effects’ ‘expected undesirable side-effects’ and ‘unknown side-effects’’ as mentioned in the MDR’ should all be understood as ‘undesirable side-effects’ and fall under the definition of an incident (Article 2(64) MDR).
(23): In this context, reference to ‘patient’ should be understood as the individual patient, i.e., acceptable in terms of the individual patient benefit.
(24): Please note that, while vigilance reporting obligations for these devices lie with the manufacturer, the sponsor may also have an obligation to report serious adverse events in the context of clinical investigations or performance studies involving CE-marked devices. For guidance on the specific requirements on safety reporting in clinical investigations or performance studies, please refer to MDC G 2020-10/1 Rev 1 and MDCG 2024-4.
(25): Article 3(3) Regulation (EEC, EURATOM) NO 1182/71 of the Council of 3 June 1971 determining the rules applicable to periods, dates and time limits, OJ L 124, 8.6.1971, p. 1. For the calculation of the period, Articles 3(4) and (5) of Regulation (EEC, EURATOM) NO 1182/71 needs to be considered, which state: ”Where the last day of a period expressed otherwise than in hours is a public holiday, Sunday or Saturday, the period shall end with the expiry of the last hour of the following working day” (Article 3(5)) and ”Any period of two days or more shall include at least two working days” (Article 3(4) of that Regulation).
(26): See Article 3(1), second subparagraph Regulation (EEC, EURATOM) NO 1182/71: “Where a period expressed in days, […] is to be calculated from the moment at which an event occurs […], the day during which that event occurs […] shall not be considered as falling within the period in question”.
(27): Article 3(2) (b) Regulation (EEC, EURATOM) NO 1182/71:”beginning of the first hour of the first day”.
(28): Article 3(2) (b) Regulation (EEC, EURATOM) NO 1182/71:“expiry of the last hour of the last day”.
(29): See Article 3(4), first subparagraph Regulation (EEC, EURATOM) NO 1182/71: “Where the last day of a period expressed otherwise than in hours is a public holiday, Sunday or Saturday, the period shall end with the expiry of the last hour of the following working day.”
(30): Please note that fields 1.2.c and 1.2.d refer to fields included in version 7.3.1 of the MIR, which is currently being finalised. Please refer to this updated version when published on the European Commission Medical Devices website: Guidance documents.
(31): Removals from the market for purely commercial non-safety related reasons are not included.
(32): The ’Union market’ refers to the territories of the European Union Member States, and due to the European Economic Area (EEA) is extended to Norway, Lichtenstein and Iceland, and via the Customs Union Agreement to Türkiye. For Türkiye, please see also the ‘Notice to stakeholders EU-Turkey Customs Union Agreement in the field of medical devices’ on the Commission website.
(33): Please note that MDCG 2022-21 ‘Guidance on Periodic Safety Update Report (PSUR) according to Regulation (EU) 2017/745’ will be revised to include IVDR aspects. Please refer to the updated version when available at the following link: Guidance documents.
(34): The manufacturer must allow 48 hours (equivalent to two weekdays) for receipt of comment on the draft FSN unless the nature of the FSCA dictates a shorter timescale e.g. for a serious public health threat.
(35): Until VGL module in Eudamed is functional, alternative means provided in the following guidance are applicable: MDCG 2021-1 Rev.1 and MDCG 2022-12 .
(36): The ‘Q&A on Practical Aspects Related to the Implementation of the Gradual Roll-out of Eudamed pursuant to the MDR and IVDR, as amended by Regulation (EU) 2024/1860,’ provides further guidance on using the VGL module, including instructions for submitting vigilance reports. Please refer to this document once it is published on the European Commission Medical Devices website: Guidance documents.
(37): OJ L 426, 29.11.2021, p. 9–15, link: https://eur-lex.europa.eu/eli/reg_impl/2021/2078.
(38): See also Article 2(23) MDR/Article 2(16) IVDR for definition of a ‘risk’. Furthermore, definition of a ‘serious risk’ is provided in Regulation (EU) 2019/1020 of the European Parliament and of the Council of 20 June 2019 on market surveillance and compliance of products and amending Directive 2004/42/EC and Regulations (EC) No 765/2008 and (EU) No 305/2011. Link: Regulation 2019/1020 – EN – EUR-Lex
(39): For information on the reporting obligations of importers and distributors, including for devices presenting a serious risk see also MDCG 2021-27 – Questions and Answers on Articles 13 & 14 of Regulation (EU) 2017/745 and Regulation (EU) 2017/746. Link: Guidance documents.
Revision History
January 2025
Redline Version
November 2024
Redline Version
February 2023
