MDCG 2021-20 Rev.0
Instructions for generating CIV-ID for MDR Clinical Investigations
Disclaimer: This document is an interactive version of the original MDCG document. We will keep it up-to-date.
This document has been endorsed by the Medical Device Coordination Group (MDCG) established by Article 103 of Regulation (EU) 2017/745. The MDCG is composed of representatives of all Member States and it is chaired by a representative of the European Commission.
Introduction
For clinical investigations under Regulation (EU) 2017/745 (MDR) the Competent Authorities (CAs) use Eudamed2 to obtain a Union-wide unique single identification number (the ‘CIV-ID’), upon submission of the required information to Eudamed2 (continuation of current MDD practice) see MDCG 2021-1 Rev.1. To do so, the below steps need to be followed:
STEP 1 – Search /generate a Clinical Investigation ID, (‚CIV ID‘)
STEP 2 – Enter the mandatory details of the related Clinical Investigation.
To allow the correct registration, search and identification of the MDR Clinical investigations in Eudamed2, 3 rules apply:
- Always start the Clinical Investigation title with “MDR” (“MDR – title”, i.e.: MDR – Detecting Traumatic Intracranial Haemorrhage with Microwave Technology)
- Always enter the Sponsor details (this is optional in Eudamed2)
- Do not indicate the risk class of the investigational device (MDR risk classes are not present in Eudamed2)
STEP 3 – Confirm the Clinical Investigation.
Instructions for generation of CIV-ID for MDR clinical investigations
These instructions cover the generation of a CIV-ID for MDR clinical investigations in Eudamed2.
The intended user is expected to have basic knowledge about general functionalities in Eudamed2, previous experience from navigating in Eudamed2, as this is not a stand-alone instruction.
The purpose of generating a CIV-ID is to create a European tracking number which can be used to identify a specific clinical investigation. A CIV-ID facilitates communication between sponsors and competent authorities, as well as between competent authorities in different member states.
As there is no possibility for sponsors to generate the Single Identification Number (SIN) foreseen in the MDR before EUDAMED is fully functional, member states may encourage sponsors to obtain a CIV-ID from a Competent Authority before the first submission of application/notification.
This would allow the sponsor to use the CIV-ID throughout their clinical investigation documentation wherever the SIN would have been used if EUDAMED was available.
The CIV-ID may also be generated by the Competent Authority upon receipt of a first submission.
It is important that the generated CIV-ID is communicated to the sponsor so that it can be used for subsequent submissions to other member states.
Search for CIV-ID
Before generating a new CIV-ID, make a search to ensure that a CIV-ID has not already been generated by another competent authority for this clinical investigation
Go to search for CIV ID: Medical Device Management > CI > CIV-ID
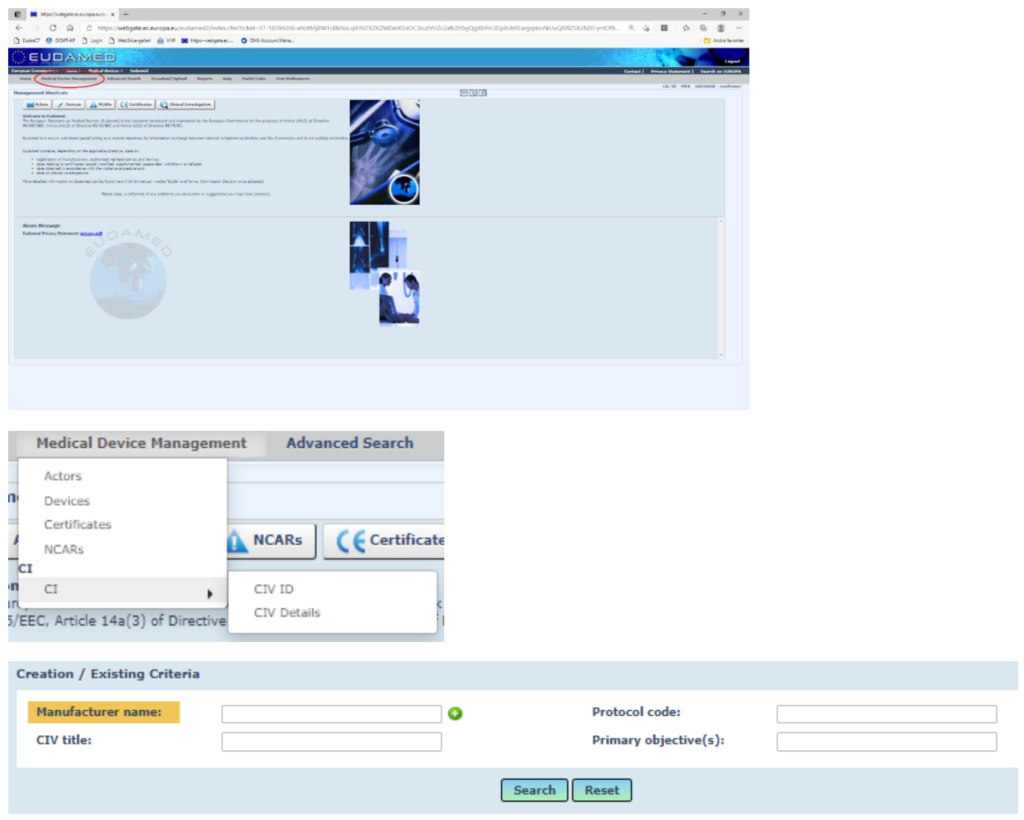
Enter the following criteria:
- Manufacturer name
- CIV title
Note that for MDR applications: Always start the CIV title with “MDR – ”(i.e.: MDR – Detecting traumatic Intracranial Hemorrhage with Microwave Technology, MDR – xyz) - Protocol code
- Primary objective(s)
Click the SEARCH button
If the search button is not visible, drag down the list below and the search button will be visible.
NOTE: If there is already a CIV-ID assigned for the clinical investigation there is no need to follow the steps below. Just note the existing CIV-ID.
If the search outcome is “No matching records found” move on to the step Generate CIV ID
Generate CIV ID
Click on the button in the upper right corner of the search field to add CIV ID.
Prefilled fields will be shown according to previous search.
Click GENERATE CIV ID
A new line will be displayed including the assigned CIV ID in draft format.
If the new line does not appear below, this can be solved as follows:
Go to search for CIV ID: Medical Device Management > CI > CIV-ID
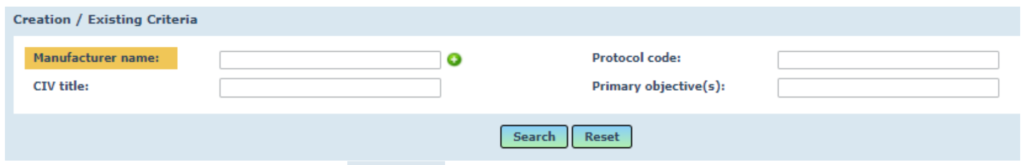
Leave all fields blank and click the SEARCH button.
The relevant Clinical Investigation will now be visible in draft format.
NOTE: Generating a CIV-ID is not enough as it will remain draft and so not visible for the other Competent Authorities. It is thus necessary to also complete the subsequent steps (i.e. to amend certain CIV-details manually and confirm the CIV-ID).
MDR-specific procedure to amend the CIV details
A limited number of CIV details will have to be entered manually, follow this procedure:
Click on the green Add (plus) button next to the draft clinical investigation to display the tabs for clinical investigation details.
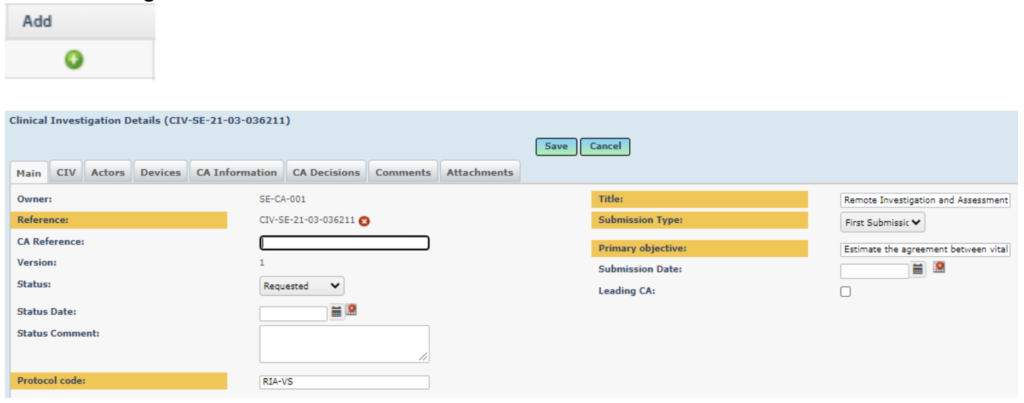
Note that there is no need to enter all details for a Clinical Investigation under MDR – the purpose of registering the Clinical Investigation in Eudamed2 under MDR is only to generate the CIV -ID.
Some of the information is prepopulated from the CIV ID generation, for example Reference, Protocol Code, Title, Submission type and Primary objective.
However, before the confirmation of an MDR-CIV-ID the following information is mandatory to enter:
In the Actors tab:
- add manufacturer if not already prepopulated by searching on the manufacturer´s name. If the manufacturer is missing in the system a new actor must be created before moving on with this addition (see section 5)
- indicate Sponsor details. Although it is an optional field in Eudamed2, it is necessary to enter Sponsor details as the sponsor is the formal applicant under MDR (not the manufacturer)
In the Device tab:
- add new device by clicking
twice and check that Clinical investigation is prepopulated as device type
- indicate device name (It is mandatory to enter at least Trade name or Generic name).
- Click SEARCH
NOTE: Never indicate the risk class for the investigational device (Eudamed2 does not accommodate MDR classes)
In the device listing click the intended use field to add this information

In the CA Information tab:
- enter CA contact (kp.central@lakemedelsverket.se)
Confirm the CIV-ID
When all mandatory information is completed click SAVE
The CI is now displayed as Proposed.
Use the confirm button to change status from Proposed to Confirmed.
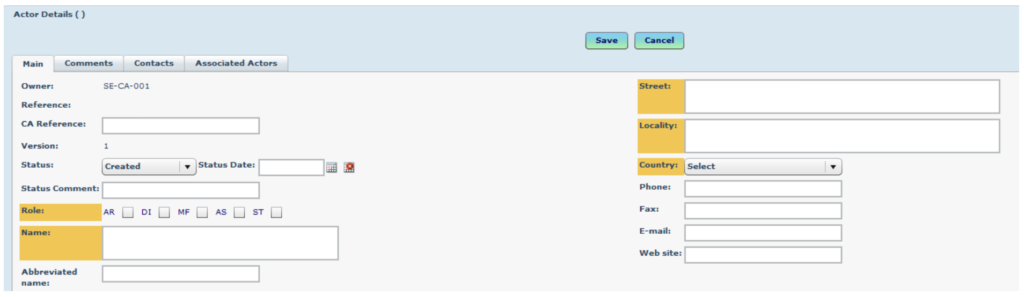
To add a new actor (to be performed only if the manufacturer is not already in the system)
Go to Medical Device Management > Actors.

Click on the button to add a new actor.
Complete all mandatory fields (yellow). Choose “MF” for manufacturer.
Click and the new actor will be shown as Proposed.
Use the confirm button to change status from Proposed to Confirmed.
It will now be possible to add the manufacturer to the clinical investigation. Go back to Section 3 and complete the Actors tab.
