MDCG 2020-18 Rev.0
MDCG Position Paper on UDI assignment for Spectacle lenses & Ready readers
Disclaimer: This document is an interactive version of the original MDCG document. We will keep it up-to-date.
This document has been endorsed by the Medical Device Coordination Group (MDCG) established by Article 103 of Regulation (EU) 2017/745. The MDCG is composed of representatives of all Member States and it is chaired by a representative of the European Commission.
Article 27 of Regulation (EU) 2017/745 on Medical Devices (MDR) introduces a Unique Device Identification (UDI), which among other functions aims to improve the identification of devices and enhance the effectiveness of post-market safety-related activities for devices. This position paper is intended to provide clarification on UDI assignment obligations for manufacturers of Spectacle lenses & Ready readers. It should be read in conjunction with the relevant provisions of Regulations (EU) 2017/745 (notably Chapter III and Annex VI) and related UDI guidance documents.
Article 10 (7) MDR mandates that manufacturers shall comply with the obligations relating to the UDI system referred to in Article 27 and with the registration obligations referred to in Articles 29 and 31 of the MDR. Article 27(3) MDR mandates that before placing a device, other than a custom-made device, on the market, the manufacturer shall assign to the device and, if applicable, to all higher levels of packaging, a UDI.
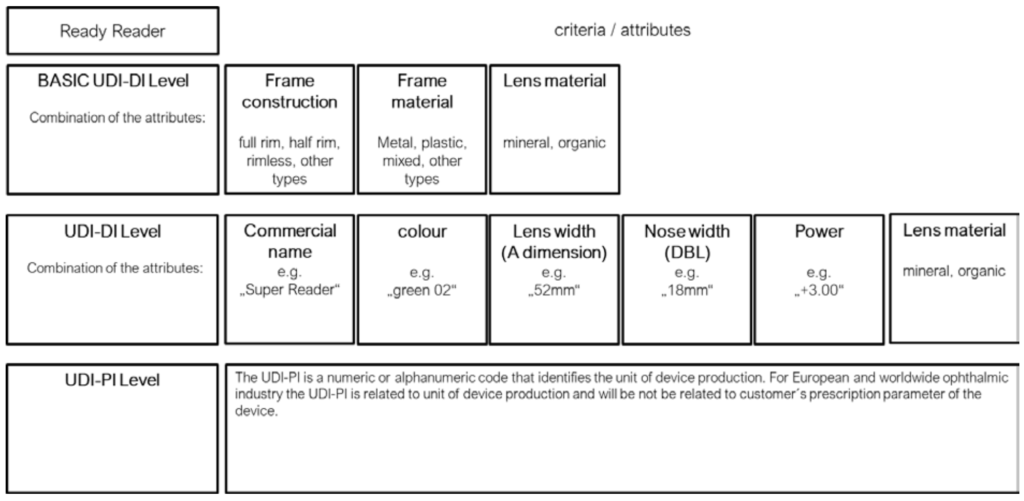
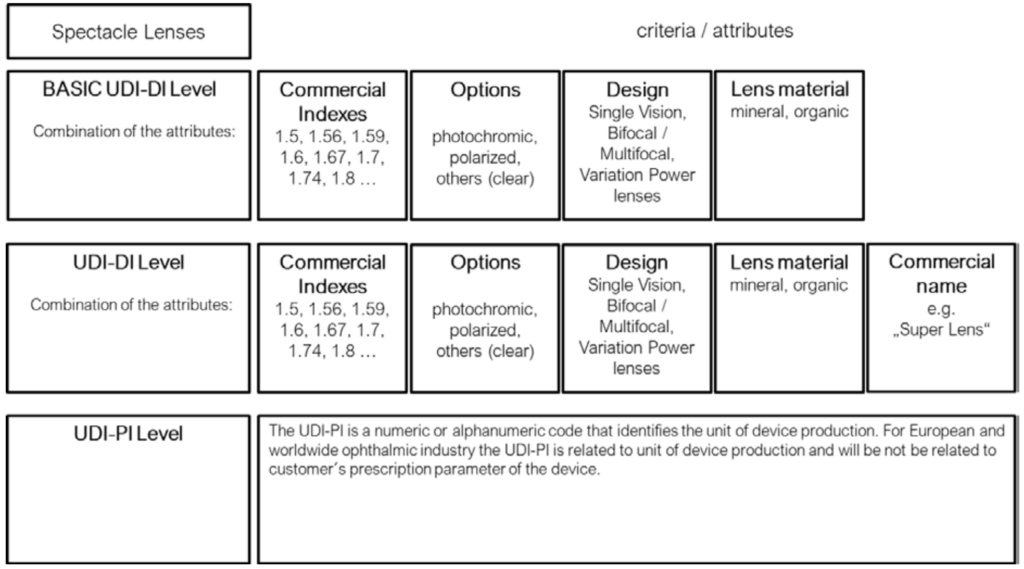
In accordance with the Basic UDI-DI and UDI-DI provisions of the MDR, triggers for both a new Basic UDI-DI and UDI-DI are defined. In particular, a new UDI-DI shall be required in the case of any change related to elements set out in Annex VI Part, C, 3.9, MDR to avoid misidentification of the device.
Bearing in mind the requirement for appropriate and uniform identification, taking into account the nature of Spectacle lenses and Ready readers and the interest of proportional data entries in EUDAMED, data elements applicable to these products and their potential triggers have been defined accordingly. Annex I & II of this document outline the Basic-UDI-DI and UDI-DI groupings to be assigned by manufacturers of spectacle lenses and ready readers.
Annex I - UDI assignment for Spectacle lenses
Annex II - UDI assignment for Ready readers